freundlich and langmuir adsorption isotherms|(PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS : Clark The data were correlated using four adsorption isotherms: Langmuir, Freundlich, and the two isotherms proposed in the IAEA Tecdoc-978. The Langmuir and Freundlich .
Goods and Services - Advertisement (Submissions) Project Description . Submitted Name . Courier Services for the Door-to-Door Delivery of DPWH Official Documents for CY 2024. 2024-08-01 15:55 : bucsitmcd: Supply/Delivery of Printer Supplies for use in the Maintenance Section, DPWH-SC2nd DEO, Koronadal City, South Cotabato. 2024-08-01 .
PH0 · Surfactant Adsorption Isotherms: A Review
PH1 · Modelling and Interpretation of Adsorption Isotherms
PH2 · Langmuir
PH3 · Guidelines for the use and interpretation of adsorption isotherm models
PH4 · Guidelines for the use and interpretation of adsorption isotherm
PH5 · Gibbsian interpretation of Langmuir, Freundlich and Temkin
PH6 · Freundlich equation
PH7 · Freundlich Isotherm
PH8 · Adsorption isotherm models: Classification, physical meaning
PH9 · Adsorption Isotherms and Kinetic Models
PH10 · Adsorption Isotherms
PH11 · (PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS
PH12 · (PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS
Google's service, offered free of charge, instantly translates words, phrases, and web pages between English and over 100 other languages.
freundlich and langmuir adsorption isotherms*******Adsorption isotherms have been of immense importance to research dealing with environmental protection and adsorption techniques. The two primary methods used for predicting the adsorption capacity of . We can model the equilibrium adsorption data by the isotherms, and investigate the adsorption information, such as the adsorption mechanisms, the . Freundlich adsorption isotherm model describes the reversible and non-ideal adsorption process. Unlike the Langmuir isotherm model, Freundlich model is not . The Redlich-Peterson isotherm is a mix of the Langmuir and Freundlich isotherms. The numerator is from the Langmuir isotherm and has the benefit of . Freundlich adsorption isotherm model is a type of isotherm model in which the absorbates form a monomolecular layer on the surface of the absorbent .The data were correlated using four adsorption isotherms: Langmuir, Freundlich, and the two isotherms proposed in the IAEA Tecdoc-978. The Langmuir and Freundlich . Adsorption Isotherms and Kinetic Models. Chapter. First Online: 27 April 2024. pp 135–154. Cite this chapter. Download book PDF. Download book EPUB. .
In the research and practice of adsorption equilibrium, a long-standing challenge is how to reconcile the classical models proposed by Gibbs, Langmuir, . Freundlich isotherms are often used to describe adsorption equilibria between a membrane and a feed solution. This is essential for the description of .The Freundlich equation or Freundlich adsorption isotherm, an adsorption isotherm, is an empirical relationship between the quantity of a gas adsorbed into a solid surface and .
During the adsorption process, adsorption isotherm is the quantitative measurement of its equilibrium and is useful in designing an adsorption system. The adsorption isotherms also describe the adsorbate and adsorbent interaction in solution. Langmuir (Papers I–V), Freundlich (Papers I–V), Temkin (Papers I–IV), and Elovich (Paper III) were the .
freundlich and langmuir adsorption isothermsDuring the adsorption process, adsorption isotherm is the quantitative measurement of its equilibrium and is useful in designing an adsorption system. The adsorption isotherms also describe the adsorbate and adsorbent interaction in solution. Langmuir (Papers I–V), Freundlich (Papers I–V), Temkin (Papers I–IV), and Elovich (Paper III) were the .
In 1916, Irving Langmuir presented his model for the adsorption of species onto simple surfaces. Langmuir was awarded the Nobel Prize in 1932 for his work concerning surface chemistry. He hypothesized that a given surface has a certain number of equivalent sites to which a species can "stick", either by physisorption or chemisorption.His theory began .

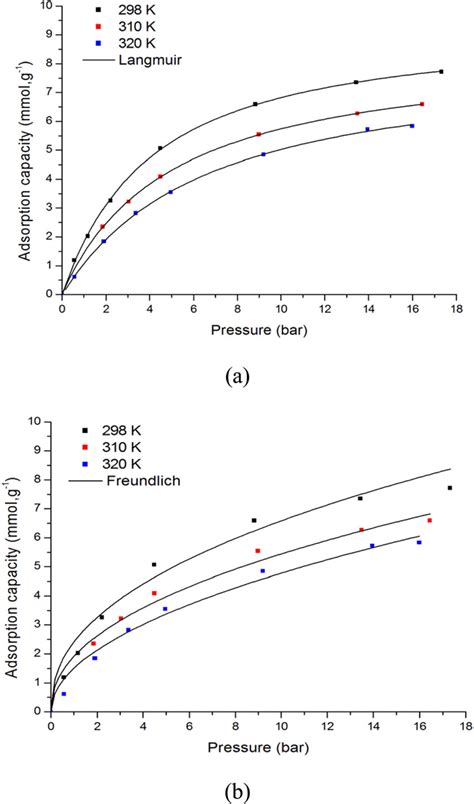
Freundlich and Langmuir Adsorption Isotherms Freundlich proposed an empirical connection between the quantity of gas adsorbed by a unit mass of solid adsorbent and pressure, However Langmuir suggested the notion of gas adsorption on a solid's surface to be formed of elementary sites.
Freundlich and Langmuir Adsorption Isotherms Freundlich proposed an empirical connection between the quantity of gas adsorbed by a unit mass of solid adsorbent and pressure, However Langmuir suggested the notion of gas adsorption on a solid's surface to be formed of elementary sites.
Experimental data from tests of orthophosphate adsorption onto oyster shell powder were fitted to the linear forms of isotherms (Eqs. (2), (9)), which are graphically represented in Fig. 2.The results indicate that the Freundlich equation, rather than the Langmuir equation, provided a good mathematical model to describe the .Adsorption Isotherm is a graphical representation of the adsorption rate of the adsorbate on the surface of the adsorbent with changing pressure under constant temperature. The primary methods used to calculate the capacity of material adsorption are Freundlich and Langmuir adsorption isotherms. What is adsorption?
The Redlich-Peterson isotherm is a mix of the Langmuir and Freundlich isotherms. . where q MLF is Langmuir-Freundlich maximum adsorption capacity (mg g −1), K LF is equilibrium constant for heterogeneous solid, and M LF is heterogeneous parameter and it lies between 0 and 1.
Freundlich's adsorption isotherm is used in this study and is described as follows [12, 13] (refer to Patiha et al.): (2) where C is the concentration in solution at equilibrium (mg/L); K F and 1 .freundlich and langmuir adsorption isotherms (PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS Majority of these models are extensions of single-component adsorption isotherms (Langmuir, Freundlich, Toth, Dubinin-Radushkevich, Dubinin-Astakhov, Do and Do, Cooperative Multimol. Sorption, etc.) or use them as inputs. Therefore, the accuracy of single-component adsorption isotherms is crit. to the reliability of the . The profile obtained from the study of concentration at different temperatures was used to obtain Langmuir and Freundlich adsorption isotherms by using well-known adsorption isotherm equations [29], [30].In both the cases linear plots were obtained, which reveal the applicability of these isotherms on the ongoing adsorption process.Freundlich isotherm does not explain this observation and therefore, fails at high pressure. The Freundlich isotherm was followed by two other isotherms – Langmuir adsorption isotherm and BET adsorption isotherm. Langmuir isotherm assumed that adsorption is monolayer in nature whereas BET isotherm assumed that it is multi-layer.
To develop an adsorption operation, in discontinuous batch or in fixed bed systems, the first step is the adsorbent choice and the second is the obtainment of the adsorption isotherms. Adsorption isotherms are a relation between the amount of adsorbate adsorbed in the adsorbent (q e) and the amount of adsorbate remaining in the .
and Freundlich isotherms with the numerator of Langmuir and is capable of approaching Henry's region when the dilution is infinite (Davoudi nejad and Ghorbanian, 2013;
The Redlich-Peterson isotherm is a mix of the Langmuir and Freundlich isotherms. The numerator is from the Langmuir isotherm and has the benefit of approaching the Henry region at infinite dilution . This isotherm model is an . Langmuir, however, was the first to introduce a clear idea of homogeneous monolayer adsorption. 4,5 There are several other adsorption isotherms reported in the literature and utilized to fit data from static adsorption tests, such as Freundlich, Redlich–Peterson, Sips, Temkin, etc. These adsorption models are discussed in a . Freundlich Adsorption Isotherm: In 1909, Freundlich expressed an empirical equation for representing the isothermal variation of adsorption of a quantity of gas adsorbed by unit mass of solid adsorbent with pressure. This equation is known as Freundlich Adsorption Isotherm or Freundlich Adsorption equation or simply .
(PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS ABSTRACT. Adsorption is a physicochemical phenomenon important in both natural and engineering processes. In the research and practice of adsorption equilibrium, a long-standing challenge is how to reconcile the classical models proposed by Gibbs, Langmuir, Freundlich, and Temkin for interpreting experimentally obtained .
An Adsorption isotherm is a relationship between a gas’s equilibrium pressure and the amount of gas adsorbed on a solid adsorbent at a fixed temperature. The extent of adsorption is determined by the temperature and saturated pressure or equilibrium pressure. A curve or plot generated by plotting the extent of adsorption . Freundlich and Langmuir adsorption isotherms are mathematical models that describe the adsorption behavior of solutes onto a solid surface. Freundlich isotherm allows for multilayer adsorption and non-uniform surfaces. In contrast, Langmuir isotherm assumes monolayer adsorption on a homogeneous surface with a fixed number of sites.
Sunny, a brilliant small-time artist is catapulted into the high-stakes world of counterfeiting when he creates the perfect fake currency note, even as Michael, a fiery, unorthodox task force officer wants to rid the country of the counterfeiting menace. In this thrilling cat-and-mouse race, losing is not an option!
freundlich and langmuir adsorption isotherms|(PDF) REVIEW OF ADSORPTION ISOTHERMS MODELS